The Role of Plastics in Shaping Modern Society #
Today’s world is surrounded by a vast array of materials, with plastics standing out due to their versatility, affordability, and adaptability. From the Stone Age to the Iron Age, humanity has evolved its use of materials, but it was not until the mid-20th century that the widespread adoption of polymer plastics truly transformed daily life. We now live in what can be called the “Plastic Age,” often without even realizing it.
The Advancement of Polymer Technology #
Plastic materials are a product of the chemical industry’s innovation. The journey begins with the petrochemical process—cracking, separating, and purifying light oil from crude oil to create various monomer raw materials. These monomers are then chemically synthesized and processed into a wide range of plastic products. Plastics are not only lightweight and durable but also offer high quality at a low cost, making them indispensable in modern life. Their properties—such as durability, flame resistance, electrical insulation, and rigidity—have led to a continuous increase in global demand.
With ongoing advancements in polymer chemistry, researchers and major brands have developed diverse polymer materials by synthesizing different monomers. These include elastomers (rubber), fibers, and plastics, each tailored for specific applications. Examples include ABS (universal plastics), PA (nylon-engineering plastics), fiber-reinforced plastics (FRP), and various additives (carbon black, plasticizers, stabilizers, antioxidants, flame retardants, UV inhibitors, etc.). Through professional processing, these materials can be customized to meet a wide range of functional requirements.
Understanding Polymerization in Plastics #
While the basic monomer raw materials for general-purpose plastics are relatively simple, the conditions and combinations during polymerization can yield materials with vastly different properties. For instance, polyethylene (PE) can be produced as either high-density (HDPE) or low-density (LDPE) variants, each with unique characteristics. The entire process—from cracking and separation to polymerization and product manufacturing—requires specialized expertise. Nonetheless, understanding the basics of the plastics that surround us is essential common knowledge.
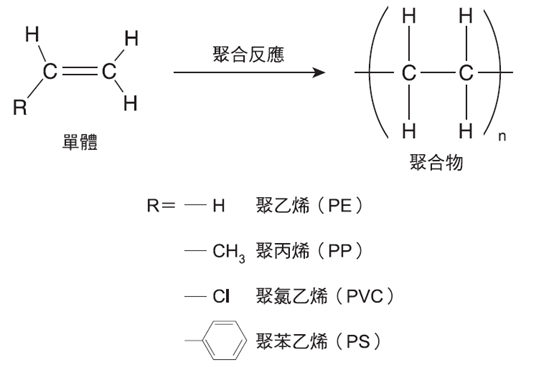
Common Plastics in Everyday Life #
The plastics most frequently encountered in daily life are numerous, but five types dominate global production and usage:
- Polyethylene terephthalate (PET)
- Polyethylene (PE)
- Polyvinyl chloride (PVC)
- Polypropylene (PP)
- Polystyrene (PS)
These are known internationally as Universal Plastics. PET, in particular, is ubiquitous—used in bottles, films, and fibers found in nearly every household. All five are high-molecular-weight linear organic materials with thermoplastic properties. They are solid at room temperature, soften and melt when heated, and can be molded into various shapes. Importantly, thermoplastic scraps and waste can be recycled and reprocessed.
At the bottom of most thermoplastic products, you’ll find internationally recognized recycling codes, which help classify materials for recycling and reuse:

Internationally recognized recycling plastic identification mark.
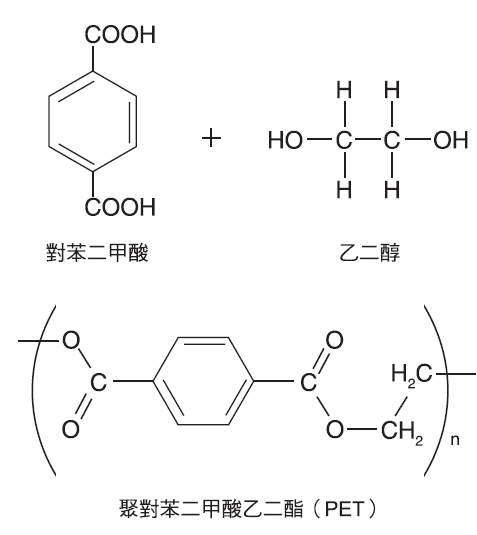
Polyethylene Terephthalate (PET) #
First produced by DuPont in the 1950s as Mylar film, PET was initially used for research, recording tapes, and X-ray films. Its rigidity, toughness, light weight, impact resistance, and chemical stability have made it a popular, cost-effective container material. However, its widespread use has led to environmental concerns, particularly regarding marine pollution.
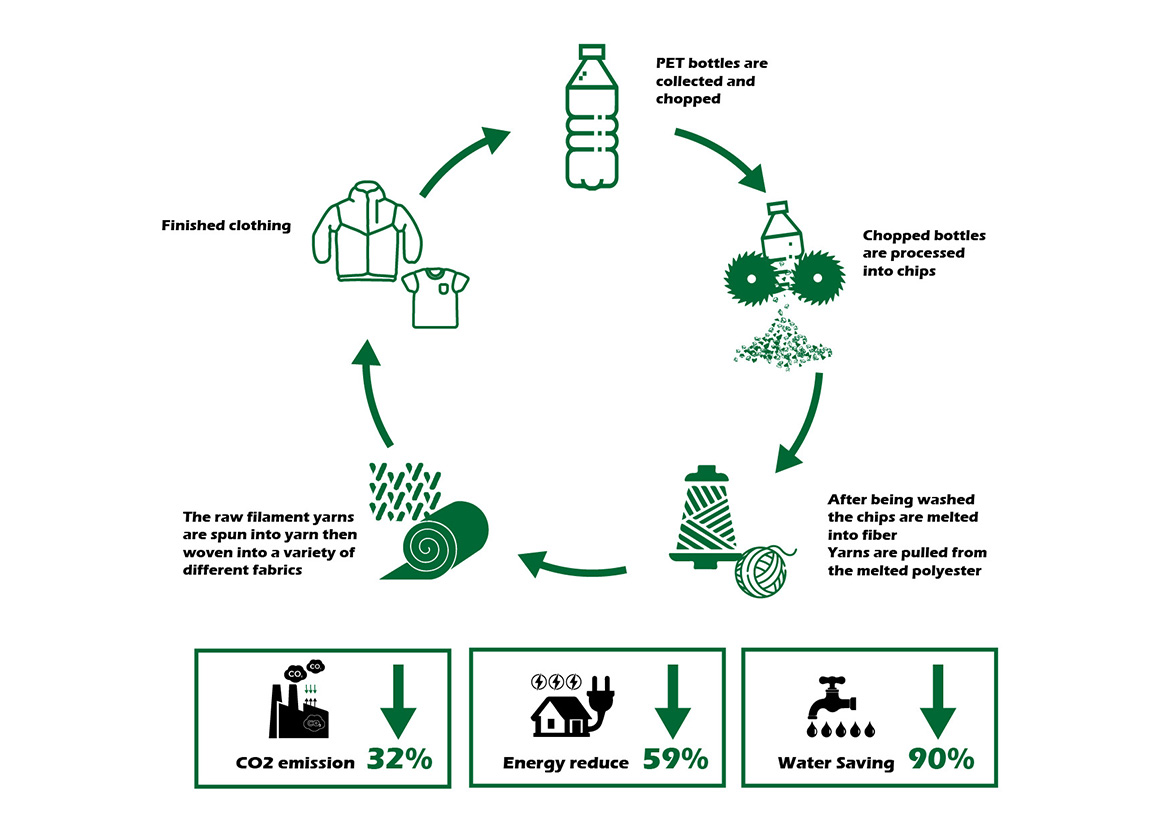
To address this, many countries promote PET bottle recycling. Recycled PET bottles are processed into PET fibers for use in fabrics, contributing to sustainability efforts. The process involves collecting, cleaning, and shredding bottles, melting the plastic into yarn, and weaving it into fabric. This recycled material is valued for its quick-drying and moisture-wicking properties and is even used in construction, such as the “Far East Ark” pavilion at the Taipei International Flower Expo.
Polyethylene (PE) #
PE comes in various molecular weights and branching structures, affecting its melting point, hardness, and transparency. Types include:
- Ultra-high molecular weight PE (used in fishing nets, industrial fabrics, parachutes)
- High-density PE (milk bottles, chemical containers)
- Medium- and low-density PE (plastic bags, packaging, food containers)
- Linear low-density and ultra-low-density PE (packaging films, plastic wrap)
Polymerization conditions and raw materials can be adjusted to produce the desired properties.
Polyvinyl Chloride (PVC) #
PVC is widely used in non-food applications such as water pipes, medical tubing, appliance wiring, synthetic leather, floor tiles, and more. It is affordable, easy to process, and naturally flame-retardant. By adding plasticizers, its softness can be adjusted, and inorganic fillers can enhance rigidity and wear resistance. However, PVC requires additives like plasticizers (commonly DEHP), stabilizers, and pigments, which can migrate to the surface over time and pose health risks. Incinerating PVC can produce toxic dioxins, raising environmental and health concerns.
Polypropylene (PP) #
PP is used in battery casings, bottles, straws, and more. Its molecular structure is similar to PE but offers superior physical and mechanical properties, including a higher melting point (130–140°C), making it suitable for steam sterilization and microwave containers. Some countries even use PP film for currency, benefiting from its oil resistance and durability.

Polystyrene (PS) #
PS is known for its low water absorption, dimensional stability, light weight, and transparency. Unfoamed PS is used in toys, stirrers, disposable cups, and appliance casings. Foamed PS (EPS or Styrofoam) is used for packaging and insulation. Due to environmental concerns, many countries have restricted its use in disposable tableware, but it remains common in aquaculture for its durability and buoyancy.
Special Considerations for PVC #
Among the five major plastics, only PVC requires significant additives, especially plasticizers like DEHP, to achieve desired properties. These additives are not chemically bonded and can migrate, posing health and environmental risks. DEHP, in particular, is an environmental hormone and can be harmful if accumulated. PVC waste cannot be easily recycled, and incineration produces toxic dioxins, making its disposal a significant environmental issue.
Important Safety and Environmental Notes #
While these five plastics are convenient, durable, and affordable, it is crucial to use them responsibly to avoid harm to health and the environment. Key points to remember:
- Thermoplastics with low melting points (except HDPE and PP) can deform or melt at high temperatures (above 60–80°C) and should not be exposed to heat.
- Plastics are flammable and should not be exposed to open flames. Burning PVC, in particular, releases toxic dioxins.
- Avoid contact with organic solvents or oils, as many plastics (PET, PS, PVC) can dissolve in substances like acetone or toluene.
Taiwan, despite lacking natural oil resources, has become a leader in plastic material technology, with robust raw material sources and advanced application technologies. Yeh Her Yow Company leverages decades of experience in research, development, and production to offer innovative solutions in the plastics field.